Hyperpolarized 129Xe MR imaging of lung
Magnetic Resonance Imaging (MRI) has a range of applications in medical diagnosis, and more than 25,000 scanners are estimated to be in use worldwide. However, lung imaging suffers from some technical challenges, limiting its application in pulmonary disease diagnosis and treatment.
Due to low proton density, movement and high susceptibility difference between air and tissue, conventional proton MRI struggles to image lung tissue and function. These can be partially overcome by the introduction of contrast agents into the lung. Hyperpolarised gases are promising contrast agents for imaging lung structure and function. The two most common gasses are helium (3He) and xenon (129Xe) isotopes. Helium isotopes are challenging and expensive to obtain, and do not provide significant functional readouts. Xenon can be challenging to work with, but promises novel physiological measurements not previously feasible. This resulting technique, termed hyperpolarized 129Xe MR imaging, has revolutionised the field of functional lung imaging.
Five years ago, an Australian Research Council (ARC) grant was awarded to researchers at Monash Biomedical Imaging (MBI) and ANSTO to design and build a machine that could reliably produce hyperpolarized xenon. In this project, Dr Wai Tung Lee and NIF Facility Fellow Dr Gang Zheng have teamed up to develop a new multimodal technology capable of high-quality investigations of the human lungs. While Dr Lee, of ANSTO, was responsible for the construction of the polarizer and production of polarized 129Xe for MR imaging, Dr Zheng, of Monash University, designed the MR experiments and adapted imaging protocols.

Figure 1. Schematic diagram of the polarizer system. Note: Some details omitted for clarity.
Hyperpolarized 129Xe (HP-Xe) gas was generated in a custom designed and constructed SEOP system at Monash Biomedical Imaging (Figure 1 and Figure 2). The system consists of a gas-polarizing unit and a gas injection and recycle unit. The core component of the gas-polarizing unit is an optical pumping cell (OPC) where the gas is polarized. The OPC is placed in an oven to regulate the amount of Rb vapour by maintaining a constant temperature between 60ºC-90ºC. The optical pumping process uses two 240W narrow-bandwidth lasers tuned to the Rb D1 transition wavelength of 794.7nm. Additional optics condition the laser light to circular polarization and shape the laser profile to fully illuminate the OPC. HP-Xe is produced in batches rather than using a continuous-flow process.

Figure 2. The spin-exchange-optical pumping system. A. Gas polarizing unit in Room 1; B. Gas injection and recycling unit in Room 2.
MR experiments were performed on a clinical 3T whole-body system (Skyra, Siemens Medical Solutions, Erlangen, Germany) with a broadband RF amplifier. HP-129Xe MRI was enabled using a bird-cage transmit/receive chest coil (RAPID Biomedical GmbH, Wuerzburg, Germany). A 2D-GRE sequence for X-nuclei MRI was used to image HP-Xe gas in lung. Proton channel images were acquired for the localization of the lung. The resonance frequency of 129Xe was set to 34.09 MHz on the 3T scanner. The human subject first quickly flushed his lung with pure nitrogen, and then immediately inhaled HP-Xe in the Tedlar bag. After inhalation of HP-Xe, the subject held their breath during the scan. The total imaging time was less than 10s.
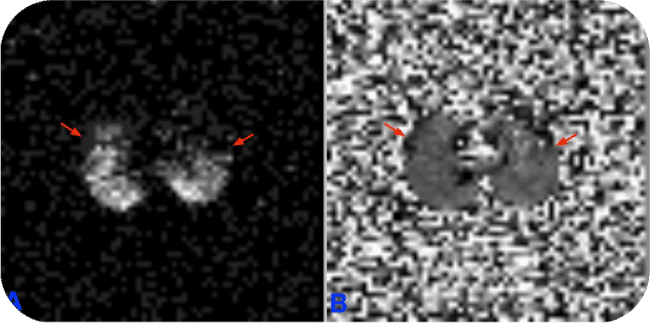
Dr Zheng outlines the results to date, “We have successfully imaged gas-phase HP-129Xe in a range of phantoms, such as the Tedlar bag, syringe and the glass cell (Figure 3). We repeated the gas-phase imaging in both ex-vivo (Figure 4) and in-vivo lamb lungs (Figure 5). Imaging results supported that our polarizer can provide sufficient polarization for lung imaging. In 2019, we successfully performed a human experiment and a gas signal from the lungs was clearly identified (Figure 6). In addition, all studied showed a clear Rf peak of gaseous and dissolved xenon which should allow us to develop methods to assess lung function. We plan to study human pulmonary disease in the near future and hope to find additional collaborators to help translate this modality into clinical use.”
Construction of the hyperpolarizer is complete; it can routinely produce HP-129Xe for research use. The technique has successfully imaged HP-129Xe in phantoms, ex-vivo and in-vivo lamb lungs and a human lung. If you are considering lung research, please see more on the Monash website, or get in touch with Dr Gang Zheng to discuss your project needs.
Publications:
Zheng G, Lee WT, Tong X, et al., A SEOP Filling Station at the Monash Biomedical Imaging Centre. Conference on polarization in Noble gases (PiNG), 2017.
Zheng G, Lee WT, Tong X, et al., Phase imaging of hyperpolarized 129Xe gas in a human lung. ISMRM 2020, submitted on 6 Nov 2019.
Acknowledgements:
National Imaging Facility, ARC (Grant LE130100035); NHMRC (Grant APP606944); CASS Foundation; Monash Biomedical Imaging, ANSTO
This story was contributed by Dr Gang Zheng of the Monash University NIF Node.