Better detection and treatment of dementia
Biogen’s Aducanumab (Aduhelm) is the first disease modifying therapy for AD approved by the The United States Food and Drug Administration (FDA). NIF’s positron emission tomography (PET) imaging facilities at the University of Melbourne, HIRF and the Hunter Medical Research Institute (HMRI) supported the Australian trial recruitment of the Biogen Phase 3 trial by screening potentially suitable participants with amyloid PET scans in collaboration with the Australian Imaging Biomarkers and Lifestyle Study of Ageing at the Florey Institute of Neuroscience and Mental Health and Austin Health.
To prescribe the treatment for prodromal and early clinical AD it will be necessary to use imaging both PET and magnetic resonance imaging (MRI) and cerebrospinal fluid (CSF)/blood biomarkers to ensure that subjects being treated have AD and that side effects from Aduhelm are properly managed.
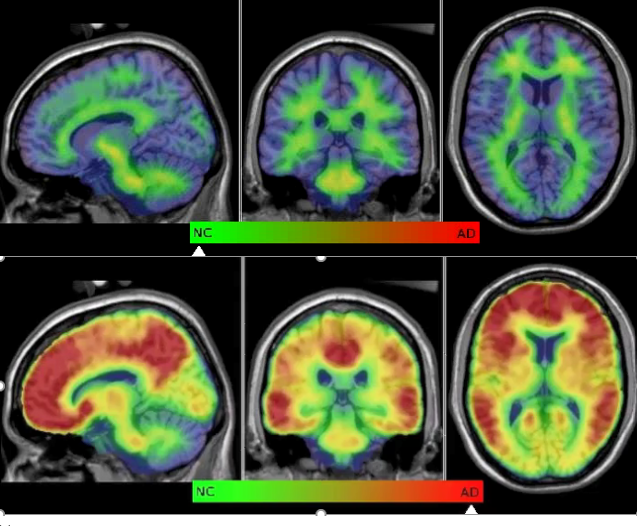
Alzheimer’s disease (AD) is the most common type of dementia making up 70% of all dementia. There are about 300,000 Australians currently living with the disease, with the average disease duration of 10 years equating to 30,000 new cases each year. These numbers are predicted to triple by 2050.
The degeneration within the brain begins two to three decades before overt symptoms, highlighting early detection is critical. NIF’s University of Melbourne Node and the Herston Imaging Research Facility (HIRF) have been involved in several dementia trials, studying different aspects of the disease including early biomarker detection, combining state of the art multimodality imaging, genetics and neuropsychology. NIF is working to assess novel radiotracers as a diagnostic tool for early detection of AD and the development of a national network of radiotracers for dementia screening in collaboration with QTRaCE and the Australia Dementia Network (ADNeT). This research aids the success of new preventative medicines, both through repurposing an existing drug and novel drug development and improves classification of AD subtypes, which impact treatment profiles.
NIF has played a major role in helping Australia to maintain its leadership in imaging applied to the dementias, particularly in AD. This has occurred at multiple sites around the country since 2012. The advent of disease modifying therapies for AD will cause an increase in demand for services and it will be crucial for new innovative and cost effective methods of service delivery in our increasingly ageing population.