First in-human images of new hope treatment for poor-prognosis prostate cancer
In a world first, supported by National Imaging Facility, Australian researchers have imaged and measured the uptake of a promising new prostate cancer therapy drug developed by Australian start-up AdvanCell, which was recently administered to its first patient in a clinical trial.
Prostate cancer is the second most common cancer for men. Around 50% of men diagnosed with local prostate cancer develop metastases, which often leads to a condition with a poor prognosis – metastatic castration-resistant prostate cancer (mCRPC) – and short survival times (a median of approximately one year or less).
Few treatments currently exist, but a new therapy has doctors very excited: targeted radioligand therapy. The ‘targeted’ therapy specifically seeks out prostate cancer cells (the job of the ‘ligand’), and the attached ‘radio’ isotope causes immense but very localised damage just to the cancer cells.
Currently, ‘beta’ radioisotopes are commonly used, which cause repairable damage to cancer cells, and can reach healthy tissue near the cancer cells. The new therapy uses a more powerful ‘alpha’ isotope that irreparably damages the cancer cells’ DNA and structures – leading to death of the cancer cell without reaching nearby healthy cells.
New isotope lead-212 sidesteps the availability problem of alpha therapies
“Alpha therapies have been used for a number of years now, and show promising results, but aren’t in large-scale clinical trials yet,” says Professor Stephen Rose of AdvanCell, Head of Translational Medicine and Clinical Science.
This is because the doses are very hard to come by. For one of the main isotopes of interest, actinium-225, Prof Rose says there are “currently only about 2000 doses available worldwide, so it’s very hard to do phase II clinical trials, and even more challenging to do larger phase III trials for drug approval.”
However, AdvanCell’s technology has thrown open a door to more hope. They created a small generator that produces clinical doses of the isotope lead-212 daily, which “solves the scalable manufacturing problem”. Because of its shorter half-life (approximately 11 hours) and short path length, lead-212 is potentially also safer for patients.
This theranostic (simultaneous diagnostic + therapy) approach dramatically reduces the side effects people normally associate with chemotherapy.
This April, 75-year-old Gary was the first person in the world to complete the treatment as part of AdvanCell’s TheraPb trial, currently enrolling at Royal Brisbane and Women’s Hospital (RBWH) and Princess Alexandra Hospital in Brisbane.
The cohort of that first trial is now full, with recruitment of the second cohort starting soon. Imaging infrastructure at Herston Imaging Research Facility (HIRF), supported by NIF, is being used to help select and measure tumour response at RBWH.
The trial is the first phase I clinical trial of a lead-212-based prostate-specific membrane antigen (PSMA) radioligand therapy for mCRPC using an Australian-produced alpha isotope.
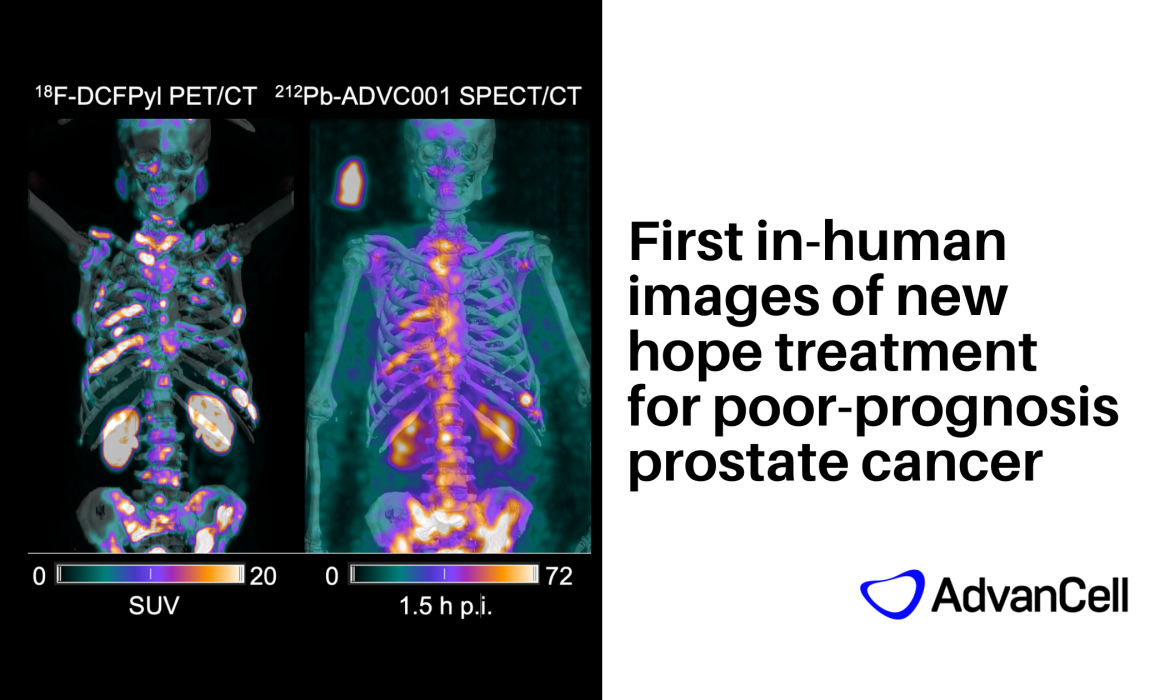
Because ‘seeing’ changes everything: first ever human SPECT/CT images
Monitoring a patient’s treatment is a huge part of any new clinical trials – researchers are looking to confirm that the targeted alpha therapy has reached the tumour sites. For lead-212, they had been in the dark.
When the trial was proposed, there was no imaging available for that isotope. There were many challenges to overcome. “It just wasn’t thought to be possible,” says Associate Professor David Pattison (Deputy Director and Nuclear Medicine Specialist, Department of Nuclear Medicine and Specialised PET Services, RBWH).
But – the medical physics team at RBWH team persisted, and showed the feasibility, clarity and benefit of imaging lead-212. Their groundbreaking work has been acknowledged by the international imaging community, and was published in The Journal of Nuclear Medicine this year.
“We showed AdvanCell that lead-212 was able to be imaged with sufficient clarity that they put it into the study,” says Dr Matthew Griffiths, Lead Nuclear Medicine Physicist at RBWH. “We ended up getting to the point of patient imaging in less than two years.”
“It really was a very exciting time to have that basic science discovery leading to inclusion of imaging into the first in-human trial protocol,” says A/Prof Pattison.
The implications of that are very profound, A/Prof Pattison says, because clinicians can directly estimate dosimetry (how much of the drug goes into the tumour and normal organs) instead of just monitoring patients over time after the drug is given.
“That’s the real benefit of theranostics,” he says. “Rather than just being more of a therapy trial, you also get to image where the treatment is going. This really closes the loop.”
Imaging also plays an important role in correctly ‘stratifying’ patients for clinical trials – different people with different presentations of illnesses can then be selected and receive a particular therapy based on their personal imaging data.
NIF provides “best-quality data from patients” for trials
Prof Rose emphasises that NIF’s support has been crucial for helping to acquire high-quality imaging data from the patients in the clinical trial.
“Like for many theranostic trials in Australia, NIF provided state of the art equipment, which is really important, and ensures excellence in the operation of that equipment. It’s one thing having a scanner, but it’s another thing having people who really know how to operate at that scale.”
NIF also supports the skill of the radiopharmaceutical manufacturing facilities, like Q-TRaCE at RBWH who currently manufacture the targeted alpha therapy for the TheraPb trial.
“Once you produce the isotope, it’s decaying. You have to conjugate that to the drug, do all the quality control, so it can safely be injected into a patient,” Prof Rose explains.
“That takes a lot of experience and a subject matter expertise in this space, so we’re very fortunate that NIF supports radiopharmaceutical development and manufacture in Australia, backed up by people who really know what they’re doing.”
Committed cross-disciplinary team key to success
This project has capitalised on the specialised knowledge of many disciplines. “It’s a real team effort to bring something like this to the fore. It highlights Australian innovation in theranostics development through AdvanCell’s property lead-212 isotope production technology and in-depth knowledge of radioligand discovery and development, partnering with experienced clinical trial sites with significant support by NIF,” Prof Rose emphasises.
NIF has a long history of supporting radiopharmaceutical development, through collaborations across universities, industry, private funding, and the Australian healthcare system. Within each area, people are contributing with a physics background, chemistry backgrounds, nuclear medicine technologists, engineering and medicine.
Over about a year, the team at Q-TRaCE worked with AdvanCell’s production team to ramp up production of the therapeutic radiopharmaceutical to a point of safely administering it to patients, by manufacturing it and validating its safety.
A/Prof Pattison describes the many steps: “As a multidisciplinary team we were meeting and discussing all of our perspectives with AdvanCell. From the medical perspective, this included the feasibility of the trial, the likelihood of patient response, comparisons to other therapies, but our whole team were involved in developing the imaging protocols, timing of the imaging, radiation safety, how to administer it, how to run the infusion safely… it’s such a great example of partnership and teamwork.”
Next steps: scaling and targeting other cancers
Clinicians are very excited about the possibilities from this trial. “We have a huge amount of optimism: we do need to see the results in real patients and we’re not quite there yet, but are very hopeful,” says A/Prof Pattison.
“There’s a huge amount of excitement about lead-212 as an alpha therapy treatment for prostate cancer, but also lead-212 potential relevance to other tumour types,” he says.
A/Prof Pattison says that if the trial results (likely in early 2025) are positive, it is feasible that the technology can be made scalable and applied worldwide.
“It really is potentially groundbreaking. There are a lot of other tumours that can potentially be treated using this technology. Lead-212 could be a key workhorse for all theranostics, because of potential limits to supply of many other theranostic isotopes.”
AdvanCell aims to, as soon as 2025, move next into melanoma and other cancer therapies. “The capabilities and infrastructure within NIF are really critical for moving this technology forward,” Prof Rose says. “We would like to open more clinical trial sites: Sydney, Melbourne and Adelaide. A lot of those facilities will be supported by NIF.”