NIF has helped move a new nanoparticle technology from development to testing to commercialisation
CEO Stewart Bartlett, Prof Benjamin Thierry and team; courtesy of Ferronova
After surgery, gastric and oesophageal cancers have quite poor prognoses, often due to metastases that have spread to lymph nodes. These cancers are difficult to treat because lymph nodes are not confined to specific places, and very small metastases cannot be detected with current imaging technology.
After gastric or oesophageal cancer surgery, the three-year relative survival rates in Australia are about 61% and 43% respectively. If the cancer has spread to lymph nodes, these survival rates fall to 41% and 28%.
Australian biotech company Ferronova has developed a super-paramagnetic iron-oxide nanoparticle (SPION), FerroTrace that targets receptors to find the highest-risk lymph (‘sentinel’) nodes in cancer patients. After being injected into a tumour, FerroTrace follows the same path through the lymphatic system as metastasising cancer cells, to highlight those high-risk lymph nodes for surgeons.
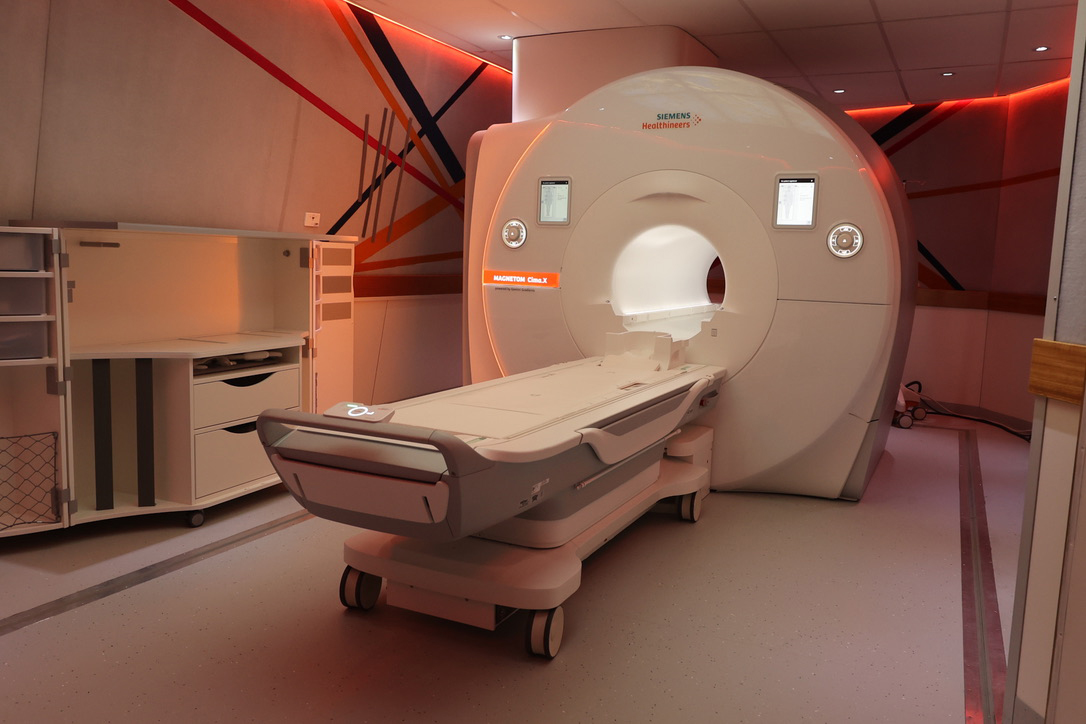
Clinicians can use MRI and a handheld surgical magnetic detector to precisely map and assess a patient’s lymph nodes, providing critical information for staging and treatment planning. Patients’ overall survival can potentially be increased if clinicians treat lymph nodes more thoroughly.
A sentinel node, or the very first draining node of a tumour bed, is representative of the overall spread of a tumour. Importantly, by sampling the sentinel node, surgeons can then determine how radical the surgical procedure has to be.
NIF continues to support Ferronova’s translation work
Ferronova Chief Executive Officer Stewart Bartlett said NIF SAHMRI (South Australian Health and Medical Research Institute) has been instrumental in supporting Ferronova to develop, test and commercialise FerroTrace.
“Ferronova’s vision is to have our SPION platform technology used on every solid tumour cancer patient that needs improved surgical staging and to enable new therapeutic options in solid tumour cancer patients,” he said.
“Our nanoparticle products have been tested in clinical trials in oral cancer, colorectal cancer, and breast cancer with a gastric and oesophageal cancer trial in progress. This translation to the clinic would not be possible without the imaging capabilities and expertise at SAHMRI’s PIRL [Preclinical, Imaging and Research Laboratories] facility and the Clinical Research Imaging Centre in Adelaide,” Mr Bartlett said.
NIF’s facilities at SAHMRI and The University of Queensland underpinned Ferronova’s preclinical imaging in small animals in 2018–19. SAHMRI’s Large Animal Research Imaging Facility supported Ferronova’s scale-up to preclinical, large-animal imaging in 2019–24 to develop surgical techniques and imaging sequences, and test biodistribution.
In 2020–22, Ferronova’s first in-human study in head and neck cancer was supported by SAHMRI’s CRIC (Clinical & Research Imaging Centre) facility – SAHMRI CRIC is the central radiology site for the ongoing gastric and oesophageal cancer trial.
The work has also been supported by electron microscopy colleagues at National Collaborative Research Infrastructure Strategy’s (NCRIS) Microscopy Australia through the University of Sydney, UNSW and the University of South Australia.
Collaboration is key to improving therapies and outcomes
The project has been driven by an engaged multidisciplinary team, with the support of radiologists, surgeons and pathologists working to improve outcomes for patients.
Dr Dwyer said the team is demonstrating the technology can improve current treatments by defining the central cancerous node, and allowing other nodes to remain untouched.
“We have showed that we can find and map sentinel nodes, the next phase is to show that it can help surgeons in theatre, and the final stage is to show that it improves patient outcomes in post-surgical morbidity or their oncological outcome,” Dr Dwyer said.
Clinical trial begins to assess overall survival and improved outcomes
The MAGMAP clinical trial (Clinicaltrials.gov ID: NCT05899985) is ongoing for patients with gastric and oesophageal cancers.
The trial is a multi-centre, partially blinded, side-by-side comparator study to assess the safety and tolerability, feasibility, and potential added diagnostic and clinical value of FerroTrace for mapping high-risk lymph nodes in subjects.
NIF’s SAHMRI facility is supporting imaging for the trial, with expansions to NIF sites at the Austin Hospital/Olivia Newton-John Cancer Research Institute and the Peter MacCallum Cancer Centre.
Eligible patients will undergo a FerroTrace injection, followed by an MRI to visualise and assess sentinel lymph nodes.

Courtesy: Ferronova
*Ferronova products are investigational use only and are undergoing clinical trials to assess their performance. They are not approved for commercial use.
About Ferronova
Ferronova is an Australian biotechnology company headquartered in Adelaide, South Australia. Shareholders include Renew Pharmaceuticals Limited, Uniseed, the University of South Australia, Artesian Venture Partners and the South Australian Venture Capital Fund (SAVCF), Powerhouse Ventures, the University of Wellington in New Zealand, the University of Sydney, PAN Ventures, Perennial Future of Healthcare Fund, and ex-Macquarie Bank executive Allan Moss. Grant assistance has been provided by the SA Government since 2016,the Federal Government’s BioMedTech Horizons Program, operated by MTP Connect, and an Australian Government CRC-P grant.