Dr Sicong Tu is excited about helping motor neurone disease (MND) patients gain a much better idea of their disease progression: “The focus of our network is on bridging the gap between leading Australian imaging researchers and leading MND clinicians,” he says.
MND is a rare disease that causes rapid deterioration and loss of function of the cells and nerves in the brain and spinal cord (motor system) that control the muscles in our bodies. This results in weakness and wasting of the muscles.
Over the past 2 years, Dr Tu (Lenity Senior Research Fellow, University of Sydney Brain and Mind Centre) has worked hard to establish the Asia-Pacific MND Imaging Initiative (AMII) network in close collaboration with researchers at The University of Queensland.
Funded through a half-million dollar grant from a FightMND-funded Collaborative Initiative Grant, AMII has set up a multisite, longitudinal research project that relies on NIF 3T and 7T advanced MRI facilities Herston Imaging Research Facility, Centre for Advanced Imaging (UQ), The Florey, University of Melbourne, Sydney Imaging (USYD), and Research Imaging NSW (UNSW), in Brisbane, Melbourne and Sydney.
AMII has three objectives: to better diagnose MND, provide clinicians with more informed predictions of their patients’ disease trajectory, and find out more about how neurodegenerative diseases like MND affect the brain.
Achieving these three objectives requires long-term, accurate imaging of as many MND patients as possible. This is no mean feat, given MND is a rare and devastating disease where there is still no effective treatment or cure: half of patients die within 3 years following diagnosis.
NIF nodes partner to collect and share critical research data
“Currently MND is a diagnosis of exclusion,” says Dr Tu. “There is no test where we can confidently say, ‘This is motor neurone disease.’ Rather, clinical diagnosis remains a process of exclusion.”
This makes it difficult for clinicians to intervene early and start to clinically monitor patients, which could improve their prognosis.
“As with all diseases, the sooner the diagnosis, the sooner clinicians can intervene to alter the disease trajectory,” explains Dr Tu.
In Australia, there are about 2,000 MND patients at any one time, due to the rapidly progressive nature of the disease. This makes it difficult to build large longitudinal imaging datasets, which could eventually result in diagnostic tests for MND and better understanding of its progression.
“Globally, the strongest MND research groups are the ones which have a national centre where every MND patient goes to be assessed, which means they can build these massive research cohorts. But that is not the case in Australia, where geographically this would be impossible,” says Dr Tu.
Instead, over the past 2 years AMII has been using NIF’s cutting-edge academic and industry experts and facilities in Sydney, Brisbane and Melbourne to collect advanced MRI brain imaging. NIF experts enable harmonised protocols to ensure scans at each centre capture using the same novel biomarker sequences of MND patients across Australia’s eastern seaboard.
“Using this approach, we have now accumulated over 500 brain scans across centres – which otherwise would take us almost a decade to collect from a single centre,” says Dr Tu.
Brain imaging reveals more about MND diagnosis and progression
Already this imaging has revealed some interesting information that was recently published in the Journal of Neurology: “When we look at the white matter fibres that connect the brain’s motor region to the spinal cord, and across brain hemispheres, we find selective patterns of neurodegeneration affected by MND,” Dr Tu says.
AMII brain scans are also being used to develop artificial intelligence (AI) disease models of MND. In a project led by collaborators at The University of Queensland, a new algorithm is being used to detect changes in tongue muscles, which could help quantify the level of damage to the lower cranial nerves. (This damage can cause MND patients to have difficulty in swallowing, chewing or even breathing.) Such information captured on brain MRI scans may be neglected when scans are analysed.
Dr Tu’s team wants to be able to improve the sensitivity of measuring such changes to create a comprehensive suite of brain-imaging tools that can be used by clinicians to better diagnose the disease and its progression.
Such tools could also help clinicians monitor the success of any treatments. Two new FDA-approved treatments have emerged in the past 2 years, following over a decade of clinical trials in MND. Brain imaging can potentially help clinicians monitor how effective the treatments are in slowing the trajectory of the disease.
“The disease is very heterogenous. For clinical care, we are also trying to understand why some patients progress much faster or slower than others: Is there a way we can tell people what to expect?” explains Dr Tu.
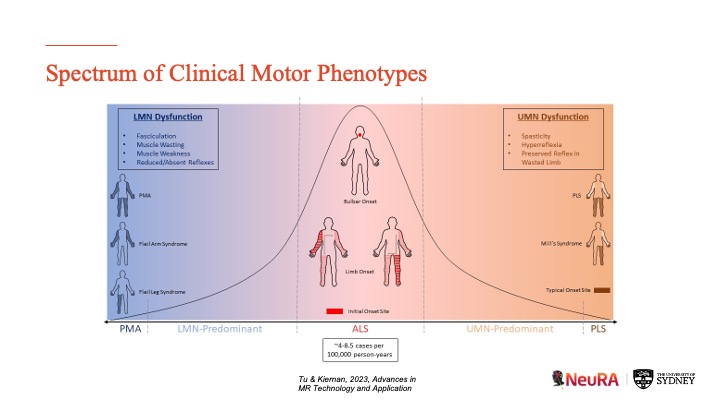
MND shares a disease continuum with other neurodegenerative disorders
Most neurodegenerative disorders have a focal point whereby disease initially spreads. Typically, by the time clinical features emerge, the disease has already spread through much of the brain.
“One of the insights from our research has shown that the neuroimaging signature of MND is mainly confined to the motor pathways of the brain through the early stages of the disease. When we measure the clinical changes of these patients on a day-by-day basis, there’s a correlation between the integrity of their motor pathways and the severity of their functional motor impairment, but not how fast the disease is progressing.”
Dr Tu explains that a ‘faster progressing’ MND is often associated with two factors: development of respiratory dysfunction (independent of brain changes), and cognitive or behavioural changes. Research from his group has previously shown clear parallels between the neuroimaging signature of brain changes in MND and dementia, finding significant associations between more widespread extra-motor changes in the brain and how aggressively a patient functionally declines.
Interestingly, some patients develop MND as well as frontotemporal dementia. In the brain scans of such patients collected by AMII, researchers are seeing extensive changes in the motor region, the frontal region as well as the temporal region.
“Looking at how the two diseases spread through imaging of patients with both diseases gives us a better understanding of how neurodegeneration spreads across the brain.”
Taking AMII beyond Australia
Now that AMII has been set up across NIF nodes in eastern Australia, researchers are looking to extend the network to other clinical sites in Australia and beyond.
These new sites would be an important addition to the AMII network: prospective validation of analysis pipelines could generate metrics useful to clinicians and contributing to AMII’s brain imaging repository.
“We are also reaching beyond Australia to engage MND research partners across the Asia-Pacific region to gauge their interest in joining the AMII network,” says Dr Tu.
Development of the AMII network is overseen by Dr Sicong Tu (Project Lead) at the University of Sydney, Prof. Matthew Kiernan (MND Neurologist; CEO) at Neuroscience Research Australia, Professor Markus Barth (UQ NIF Node Director) and Dr Thomas Shaw (Bill Gole MND Postdoctoral Research Fellow) at The University of Queensland, Prof. Robert Henderson (MND Neurologist) at Royal Brisbane Women’s Hospital, Dr Thanuja Dharmadasa (MND Neurologist) at the Royal Melbourne Hospital, and Prof. Paul Talman (MND Neurologist) at Deakin University.