The Australian National Imaging Facility and Therapeutic Innovation Australia have supported development of a promising new ovarian cancer drug from early-stage development, through preclinical studies and now its first in-human studies.
Ovarian cancer is dubbed ‘the silent killer’ due to its vague early symptoms and late diagnosis – resulting in a high mortality rate – and this is compounded by a lack of new drugs and clinical developments.
But researchers are driven to make a difference for this disease, and the nearly 2,000 women diagnosed each year in Australia.
Professor John Hooper (Cancer Biology Research Group leader, Mater Research Institute) and multiple research teams have worked for decades to develop 10D7, an enigmatically named beacon of hope for advanced and early-stage ovarian cancer patients – and potentially for those with other cancers, too.
Enabled by national research facilities such as NIF and the rich multidisclipinary collaborations across the country, this is an Australian success story from the initial discovery of the antibody to the first in-human trials.
Drug development lags because of profitability
Professor Katie McMahon, NIF’s Queensland Node Co-Director and Deputy Director of the Herston Imaging Research Facility (HIRF), explained the difficulties associated with drug development for more advanced (or rare) cancers.
“Drug development for advanced cancers, like ovarian cancer, is often less of a priority because the small patient population makes research less attractive,” she says.
In 2024, for instance, 1,805 women were diagnosed with ovarian/fallopian tube cancers, making up only 2.4% of the cancer diagnoses in women each year.
The numbers lay bare the challenge of clinical developments for more advanced/rare cancers:
| Last year | Survival rates | |
| Ovarian/fallopian tube cancers | 1,805 diagnoses | 5 years: 49% |
| Bladder cancer | 3,319 diagnoses | 5 years: 57% |
| Pancreatic cancer | 3,902 deaths | 5 years: 13% |
The current scanning and imaging methods to detect these kinds of cancers have been described as “limited and outdated”.
But our researchers, Prof McMahon explains, “are not driven by profit. They’re driven by whether it’s possible or not. They say, ‘Let’s try it, get it to human trials. Let’s give a treatment to people who need a treatment’.”
Clinical developments are “a lot more flexible when it’s driven by a need and the investigators, rather than a balance sheet,” she says. “For us, it’s about the opportunity of giving hope and new possibility to people with advanced cancers.”
New antibody targets aggressive cancers
Since 2020, Prof Hooper and his team have focused on an antibody called 10D7 that disrupts the activities of a protein called CDCP1, originally found within the most aggressive prostate cancers.
CDCP1 is an important part of signalling cascades that control how the cancer cells survive, grow, metastasise and – significantly – resist treatment. It is found in many breast, ovary, pancreas, lung, bowel, kidney, liver and blood-cell cancers.
Prof Hooper can find tumours strongly expressing the CDCP1 protein by using a tracer of low-radiation zirconium-89 (89Zr) attached to the 10D7 antibody. PET scans ‘light up’ wherever the tagged antibody accumulates in the body.
“If, from the PET scan, the levels are seen to be much higher in the tumour than in normal organs, then the clinical team and the patient can make a decision about whether to proceed to treatment,” he says.
In the treatment phase, 89Zr will be swapped for a higher energy radioactive particle, such as lutetium-177, which is referred to as a ‘therapeutic payload’.
When the therapeutic payload arrives and binds to the surface of the cancer cell (using the 10D7 antibody), it gives off radiation over a very short distance. The energy is high enough to puncture the cancer cell, break apart the cell nucleus, and kill the cancer cell.
After three to six cycles of treatment, the idea is for the team to revert the antibody payload to 89Zr, and perform another PET scan to find residual tumours and see how effective the treatment was.
First in-human trial for ovarian cancer
“We’ve just imaged our first ovarian cancer patient, and we’re also recruiting our first bladder cancer patients – who we’re hoping will be imaged in the next few weeks,” says Prof Hooper.
These first in-human trials of ‘ZOvCa’ for ovarian cancer and ‘Clearview-BC’ for bladder cancer, are testing the safety of using the 10D7 antibody to deliver 89Zr for PET-scan based detection of ovarian or bladder cancer – the essential first step of hope on the long road for advanced cancer patients.
Up to 30 advanced ovarian cancer patients will participate in PET imaging trial, and 20 people with advanced bladder cancer are also slated to be imaged in the Clearview-BC trial.
Prof Hooper’s team is also keen to perform imaging with 89Zr-labelled 10D7 on patients who have pancreatic, prostate, breast or bowel cancer – to understand if these patients could also potentially benefit from a drug that targets the CDCP1 protein.
“With that many patients generously involved in the trials, we will soon know whether 10D7 is safe to use, and about its biodistribution in tumours and normal organs. My prediction is that the agent’s going to be very safe, primarily because the dose of the protein and the radiation is so small for patients.”
National facilities have enabled entire journey to clinical trials
“Having national research facilities available has been a game changer – places like the National Imaging Facility, the National Biologics Facility, Therapeutic Innovation Australia, the National Biologics Facility, and the Herston Imaging Research Facility,” says Prof Hooper.
“Without them, I couldn’t have done what I have, and they came through at exactly the right time for the research that I was doing.”
The 10D7 antibody clinical-development process has traveled through the entire translational process for health innovation.
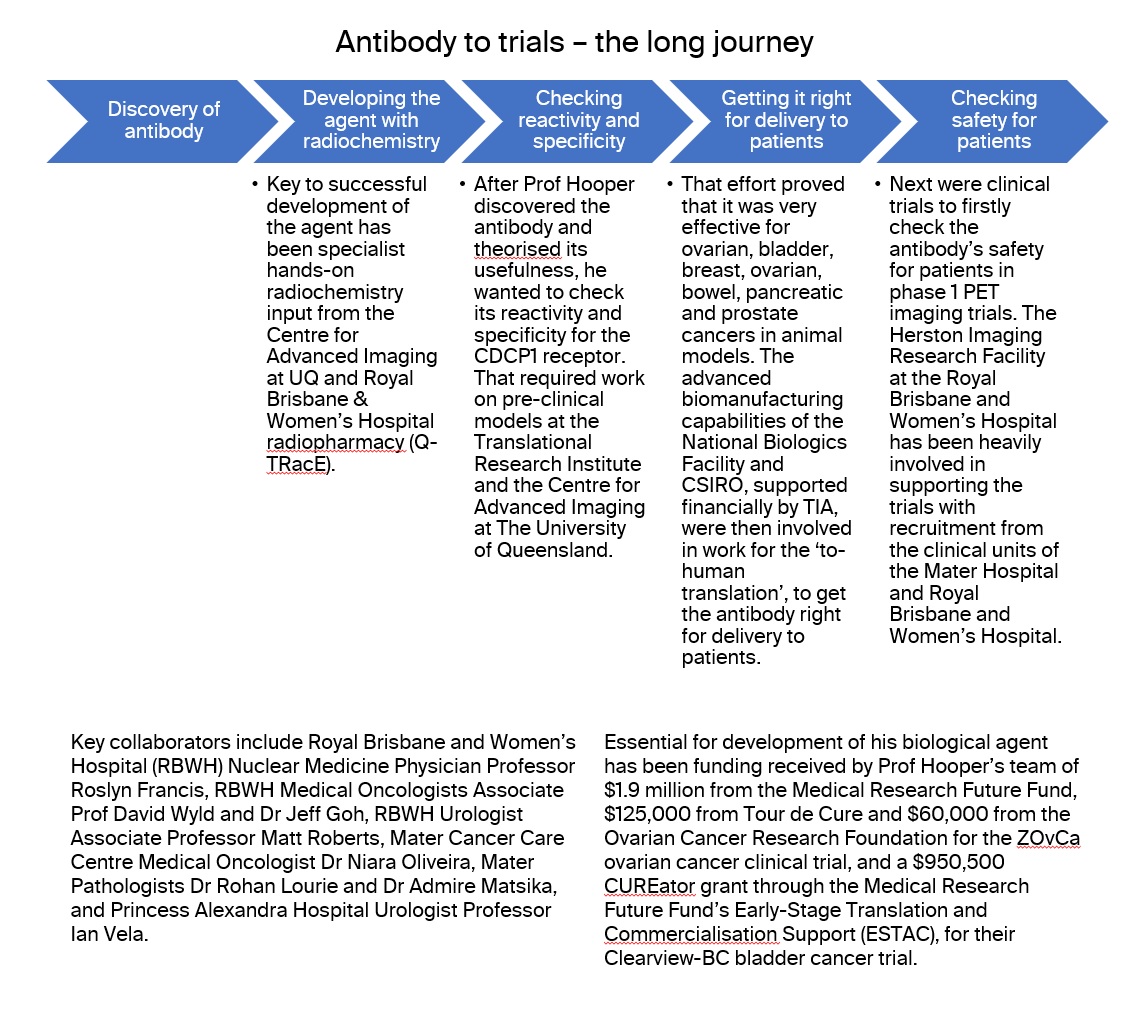
A journey of Australian effort
All the steps through to human trials have been done in Australia, apart from one toxicology experiment overseas.
“I think you could count on the fingers of one hand the number of therapeutical agents that are developed solely in Australia,” says Prof McMahon, emphasising the uniqueness of this process.
“These are the collaborations that HIRF was built for, and the sort of trials that we love to put extra effort in to support,” she says.
The national capabilities enable a seamless process without needing outside facilities.
“For someone like me, a fundamental researcher wanting to move into the translational space,” Prof Hooper says, “the more people like me who know about those facilities and can access them, the better.”
Prof Hooper has much praise for the researchers and clinical teams: “Through this process, I’ve experienced that Australians are really prepared to take on the latest innovations and do clinical trials. And if the trial data shows that something should be implemented, the clinical teams are very quick at implementing those new discoveries in the clinical practice.”
Breaking silos: a fully connected research pipeline
“People are used to new drugs always coming from overseas,” says Prof McMahon. However, with continued strong investment from NCRIS and NIF, the Australian infrastructure has developed in recent years, and strong relationships have developed.
“We have increased the connections,” she says. “All the different parts of the translational pipeline are all talking to each other and people know who to link you up with if you need help. It’s really lifted the limitations on how effective we can be.”
Benefits flowing down to early-stage patients
Next on the horizon, Prof Hooper says, are two possibilities: treatment and imaging. The treatment ‘arm’, he explains, is optimisation and commercialisation of the antibody for production and transport to increase the number of people that can be treated.
The other ‘arm’, imaging, offers hope for both early-stage patients and those with advanced cancers. At initial diagnosis, 10D7 could be used to visualise how far a cancer has spread beyond an initial organ, and inform clinicians about how best to treat the patient.
10D7 could also provide benefits for surgery: guiding surgeons doing resections using fluorescence. Instead of a radioactive particle, a fluorescent particle could be attached and injected just before surgery, indicating to surgeons any residual tumour tissue.
“The research I do,” says Prof Hooper, “is really focused on advanced stage cancers, but 10D7 absolutely has the potential to also benefit earlier stage patients.”
First image courtesy: UniQuest